Think of THCA as the quiet version of a more familiar compound. It’s a naturally occurring molecule in fresh cannabis plants that doesn’t produce the “high” most people associate with marijuana, yet it plays a foundational role in the plant’s chemistry and in how cannabis products are made, tested, and regulated. Understanding THCA helps separate fact from myth and clarifies why raw plant material, tinctures, and heated extracts behave differently.
This article,”THCA Simplified: A Clear,Neutral Clarification,” strips away jargon and hype to give a straightforward look at what THCA is,how it forms and transforms,and why it matters to researchers,consumers,and regulators. You’ll get a concise primer on the basic chemistry, a plain-English explanation of the difference between THCA and THC, and an overview of current knowledge about its biological interactions-without overstating benefits or risks.
We’ll also touch on practical implications: how THCA shows up in lab tests, why decarboxylation changes a product’s effects, and how legal frameworks treat THCA compared with THC. Wherever the science is unsettled, the article flags uncertainty rather than drawing sweeping conclusions, so you can rely on clear, balanced facts.
If you want to know the essentials-what THCA is, why it’s relevant, and what to watch for-this piece will guide you through the key points in an accessible, neutral way.
THCA Simplified A clear primer on what THCA is and how it differs from THC
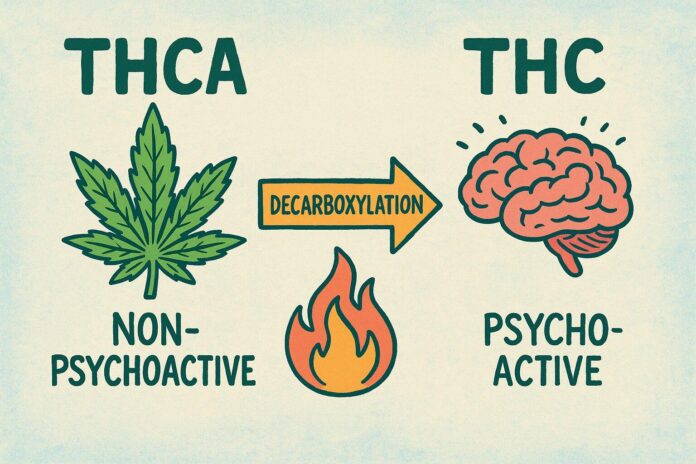
Inside fresh cannabis plant material you’ll mostly find THCA – tetrahydrocannabinolic acid – a mildly acidic precursor that the plant manufactures naturally. Think of it as the “raw” version of one of the plant’s best-known molecules: chemically very close to THC but bearing an extra carboxyl group that changes how it behaves biologically and chemically. In living tissue and unheated extracts, THCA accumulates in glandular trichomes and is the dominant form before any processing.
Were THCA and THC diverge is dramatic in practice. THCA itself is largely non-psychoactive; it doesn’t produce the intoxicating effects associated with THC until that extra carboxyl group is removed. That conversion-known as decarboxylation-happens with heat, prolonged light exposure, or aging. Key practical differences include:
- Psychoactivity: THCA – non-intoxicating; THC – intoxicating once activated.
- Activation: THCA requires decarboxylation (heat/time) to become THC.
- measurement: Labs report THCA and THC separately; some labels show “potential THC” after conversion.
- Use cases: Raw juicing or low-heat extracts preserve THCA; smoking or vaping converts it into THC.
| Property | THCA | THC |
|---|---|---|
| chemical form | Acidic cannabinoid (contains -COOH) | Neutral (after decarboxylation) |
| Psychoactivity | Generally non-psychoactive | Psychoactive |
| How it appears | Fresh flower, raw extracts | Smoked, vaped, baked products |
| Common consideration | Preservation requires low heat | Measured for potency and effects |
For consumers, cultivators, and clinicians, the distinction matters for labeling, legal interpretation, and desired effects.Laboratories often report THCA separately and sometimes calculate “converted” THC using a standard factor-review Certificates of Analysis when dosing or choosing products. If you want the non-intoxicating chemistry of the plant, seek preparations that preserve THCA; if you want the classic effects associated with cannabis, heat will reliably unlock THC through decarboxylation.

The chemistry behind THCA Decarboxylation pathways bioavailability and activation factors
At the molecular level,THCA is the carboxylated precursor of THC: it carries an extra carboxyl (-COOH) group that makes the molecule larger,more polar,and less able to cross lipid membranes. The core chemical change in activation is a decarboxylation reaction – the loss of carbon dioxide – which shifts THCA into a more lipophilic form with altered receptor affinity. This simple bond break transforms the compound’s pharmacology and physical behavior, influencing volatility, solubility, and how it interacts with biological targets.
The conversion follows several distinct pathways rather than a single route. Common mediators include thermal energy, photochemical processes, enzymatic reactions within the living plant, and slow oxidative changes during storage. Each pathway differs in kinetics and by‑products: thermal and photochemical routes are often faster and produce immediate activation, while enzymatic and oxidative routes proceed more slowly and can be accompanied by additional chemical transformations that affect purity and potency.
- Heat – promotes rapid decarboxylation and increased lipophilicity.
- Light & oxygen – can drive photochemical and oxidative changes over time.
- Matrix effects - plant material, solvents, or fats influence how readily decarboxylation proceeds.
- pH and catalysts – acid/base conditions and catalytic surfaces can alter reaction rates.
Activation state strongly impacts bioavailability and the subjective profile of effects. When the carboxyl group is removed, the molecule is generally more able to cross biological barriers, so inhalation gives fast systemic availability while oral routes introduce first‑pass metabolism that can yield active metabolites with different potency and duration.Formulation choices – such as combining with lipids,using encapsulation,or stabilizing agents - influence both the preservation of THCA and the efficiency of conversion to THC when activation is desired.Analytical monitoring (e.g., chromatography) is commonly used in research and product advancement to track these chemical transitions and guide formulation strategies.
| Pathway | Typical Outcome |
|---|---|
| Thermal | Rapid conversion to THC; increased volatility |
| photochemical/Oxidative | Slower activation,potential degradation products |
| Enzymatic (in planta) | Controlled biosynthetic balance between acids and neutral cannabinoids |

How THCA interacts with the body Endocannabinoid system pharmacology and testing limitations
THCA is the raw, acidic precursor of THC found in fresh cannabis; unlike THC, it is largely considered non-psychoactive because it binds weakly - if at all - to the brain’s primary cannabinoid receptor CB1. Studies so far suggest only modest affinity for CB1/CB2 receptors,and many of THCA’s observed actions seem to arise from indirect pathways rather than classic receptor activation. Importantly,heating (decarboxylation) converts THCA into THC,which does strongly activate CB1 and produces the familiar psychoactive effects.
Beyond cannabinoid receptors, THCA appears to interact with other molecular targets: TRP channels (transient receptor potential), PPARγ nuclear receptors, and certain metabolic enzymes such as COX isoforms have been implicated in preclinical work. These interactions suggest a pharmacological profile that can be modulatory - influencing inflammation, cell signalling and ion flux – rather than producing overt psychoactivity through central CB1 stimulation. Most evidence comes from in vitro and animal studies,so the human pharmacology remains incompletely mapped.
- Mechanism nuance: THCA likely exerts effects through multiple low-affinity targets rather than a single, high-affinity receptor.
- conversion caveat: Thermal or metabolic decarboxylation turns THCA into THC, complicating interpretation of both effects and lab results.
- Bioavailability: Oral and topical routes may limit central exposure; peripheral targets could be more relevant.
testing and measurement bring their own limits.Routine drug screens detect THC metabolites (e.g., THC‑COOH) and usually do not distinguish THCA; specialized methods like LC‑MS/MS are required to quantify THCA separately. Cross‑reactivity in immunoassays,sample handling that induces decarboxylation,and inconsistent product labelling all muddy the data.
| Pharmacology aspect | What it means |
|---|---|
| Receptor binding | Low CB1/CB2 affinity; possible non‑cannabinoid targets |
| Psychoactivity | Not typically psychoactive unless converted to THC |
| Testing | Standard screens miss THCA; LC‑MS/MS needed for accuracy |
- Clinical gaps: Limited human trials make it hard to translate lab findings into reliable outcomes.
- Analytical issues: Sample heating, storage and cross‑reactive assays can produce false positives or misclassification.
- Regulatory impact: Laws and workplace policies focus on THC/THC‑COOH, so THCA’s presence is often irrelevant unless it converts to THC.
Evidence review Potential benefits risks adverse effects and gaps in the research
The current evidence for THCA is a patchwork of laboratory experiments, animal work, and a small number of human observations. While cellular and rodent models often show biological plausibility-anti‑inflammatory, neuroprotective and antiemetic mechanisms-these findings do not automatically translate into clinically meaningful outcomes. Human data are limited and heterogeneous, so claims of clear therapeutic benefit remain tentative rather than proven.
Clinical signals that have emerged fall into a few recurring themes, usually reported in small studies or anecdotal case series rather than large controlled trials. Commonly discussed potential benefits include:
- Pain and inflammation: reduced markers of inflammation in preclinical work, occasional symptom relief reported by users.
- Nausea and appetite: early reports suggest antiemetic potential, especially in contexts where THC is undesirable.
- Neuroprotection and mood: intriguing lab data hint at protective effects on neuronal cells and modulation of mood‑related pathways.
- Sleep support: some users report improved sleep quality, though evidence is anecdotal.
Safety signals are not extensive but warrant caution. Reported adverse effects tend to be mild to moderate and often nonspecific, yet product variability complicates attribution. Typical issues include dry mouth, lightheadedness, drowsiness, and gastrointestinal upset. Meaningful risks to consider are the potential for thermal conversion of THCA to THC (producing psychoactive effects), interactions with prescription medications (CYP enzyme involvement), and contaminants from poorly regulated products (pesticides, solvents). A concise snapshot of the evidence strength is shown below.
| Evidence level | Common findings | Confidence |
|---|---|---|
| Preclinical | Mechanistic anti‑inflammatory,neuroprotective | Moderate |
| Observational | User reports of symptom relief | Low |
| Randomized trials | Very few,small and mixed results | Very low |
| Safety data | Short‑term,limited reporting | Low |
Key gaps remain: standardized dosing,consistent product characterization,robust randomized controlled trials,long‑term safety studies,and investigations into drug interactions and vulnerable populations (pregnant people,children,older adults). Priorities for future research should include:
- Placebo‑controlled trials with clear endpoints and standardized THCA preparations.
- Pharmacokinetics and stability studies to understand conversion to THC under real‑world use.
- Safety monitoring over months to years and in combination with common medications.
Legal and labeling guidance Navigating regulation testing thresholds and consumer protections
Regulatory frameworks for cannabinoid products are a patchwork: federal law defines hemp as plants and products with no more than 0.3% delta‑9 THC on a dry weight basis, but states and countries often set their own testing thresholds, labeling rules and enforcement priorities. Because THCA chemically converts to delta‑9 THC when heated or aged,regulators and testing labs frequently treat it as part of the total THC picture rather than a free-pass ingredient. For this reason, many compliance teams work with accredited laboratories that can report both raw THCA content and the converted delta‑9 equivalent (using a standard conversion factor), and they maintain clear chain‑of‑custody documentation for each batch.
Transparency on the package is essential. Labels that are easy to read and backed by a current Certificate of Analysis (COA) protect consumers and reduce legal risk. Recommended label elements often include:
- Total potential THC (THC + THCA converted to delta‑9 equivalent)
- Lab batch number and link/QR to the COA
- Net weight, cannabinoid breakdown and harvest/pack date
- Standard legal warnings (e.g., keep out of reach of children, not for sale to minors)
Testing requirements also extend beyond potency. Consumer protection rules commonly require screening for pesticides, heavy metals, residual solvents, and microbial contaminants; adequate limits and methodologies vary by jurisdiction, so brands should confirm state lists and action levels. Typical compliance checks include:
- Third‑party potency testing (THC, THCA, CBD, other cannabinoids)
- contaminant panels (pesticides, metals, solvents, microbes)
- Stability and shelf‑life considerations, especially for THCA‑rich formulations
| Label Element | example Entry |
|---|---|
| Total Potential THC | 0.28% (THC + THCA equiv.) |
| COA | Batch #A123 - QR links to lab report |
| Safety Warning | Keep away from children. Not for sale to minors. |
Practical compliance is an ongoing process: use ISO‑accredited labs where possible, keep conservative internal limits to cover inter‑lab variability, and update labeling promptly when rules change. By combining clear consumer information, robust testing for safety and potency, and meticulous record‑keeping, brands can navigate the regulatory maze while giving buyers the information they need to make informed choices.
Practical recommendations Safe use dosing product selection and questions to ask suppliers
Start low and go slow. Because THCA can convert to THC when heated, dosing depends on how you use the product. For raw or cold preparations (juices, raw tinctures) many users begin at about 1-5 mg of THCA and increase gradually; if you’re inhaling or heating flower/concentrates assume conversion to THC and begin at a much lower THC-equivalent dose (often 1-2.5 mg for sensitive people, 2.5-5 mg for most beginners). Wait at least 2 hours before increasing an edible dose and allow 15-45 minutes to assess sublingual tinctures; inhaled forms act within minutes. Never mix with alcohol or sedatives without medical advice and avoid driving or operating machinery until you know how a product affects you.
Choose products that make your decision easy: clear labeling, transparent testing and conservative manufacturing practices reduce surprises. Look for a visible Certificate of Analysis (COA) linked to the batch,a full cannabinoid breakdown (THCA vs. THC), and contaminant screens for pesticides, solvents and heavy metals. Prefer CO2 or ethanol extractions over unknown solvent methods,and favor producers who list terpene profiles,origin of hemp and a batch number for traceability.
Ask suppliers direct questions – good vendors will welcome them. Useful prompts include:
- Can you provide the COA for this exact batch?
- What are the THCA and THC amounts per gram/serving?
- Was the product decarboxylated or intended for heating?
- Which contaminants were tested and what were the results?
- What extraction method and solvents (if any) were used?
- Do you have recommended starting doses and usage guidance?
If a supplier deflects or cannot produce test data, treat that as a red flag.
| Form | Typical onset | Typical duration | Dosing tip |
|---|---|---|---|
| Raw tincture/juice | 10-30 min | 2-4 hrs | Start 1-5 mg THCA |
| Sublingual tincture | 15-45 min | 3-6 hrs | Assess after 30-60 min |
| Vape/smoked | Immediate | 1-3 hrs | Begin with a single small inhalation |
| Edible (heated) | 30-120 min | 6-12 hrs | Start low, wait 2 hrs before more |
Store products in a cool, dark place, keep out of reach of children and pets, and consult a healthcare professional if you take prescription medications or have underlying health conditions.
In Retrospect
Like the raw leaf that holds its secrets until heat unlocks them, THCA sits quietly at the edge of many conversations about cannabis – chemically distinct, biologically captivating, and surrounded by evolving science and law. We’ve pared back the jargon to show what THCA is, how it differs from THC, and why context – temperature, testing, and regulation - matters when discussing it.
If anything here is clear, it’s that our understanding of THCA is still unfolding. Researchers continue to map its chemistry and potential roles, and policymakers and consumers are adapting as new data and products emerge. For practical questions about use, testing, or legality, rely on up-to-date, reputable sources and local guidance.
Thanks for reading. Take the basics you’ve learned here as a starting point – stay curious, check the facts, and remember that in the world of cannabinoids, clarity often arrives one careful experiment at a time.